ReActiv8® Restorative NeurostimulationTM demonstrated statistically significant and clinically meaningful superiority vs. standard of care in all primary and secondary measures of disability, pain and quality of life
Safety profile consistent with prior ReActiv8 clinical trials and favourable to other neuromodulation procedures
ReActiv8 is now supported by the most complete and robust set of clinical evidence of any neuromodulation therapy for axial back pain globally.
Mainstay Medical Holdings plc today announced the publication of positive one-year primary assessment results of the RESTORE randomized clinical trial of ReActiv8 for the treatment of intractable chronic low back pain. The data show that the addition of ReActiv8 Restorative Neurostimulation therapy to current standard of care results in superior improvements in back pain-related disability, pain and quality of life compared to standard of care treatments alone. The results were published in Pain and Therapy, a leading peer-reviewed journal, and the article is available free of charge here: doi.org/10.1007/s40122-024-00689-0.
The study included 203 patients, with 99 randomized into the treatment arm and 104 randomized into the control arm. Key results from the study include:
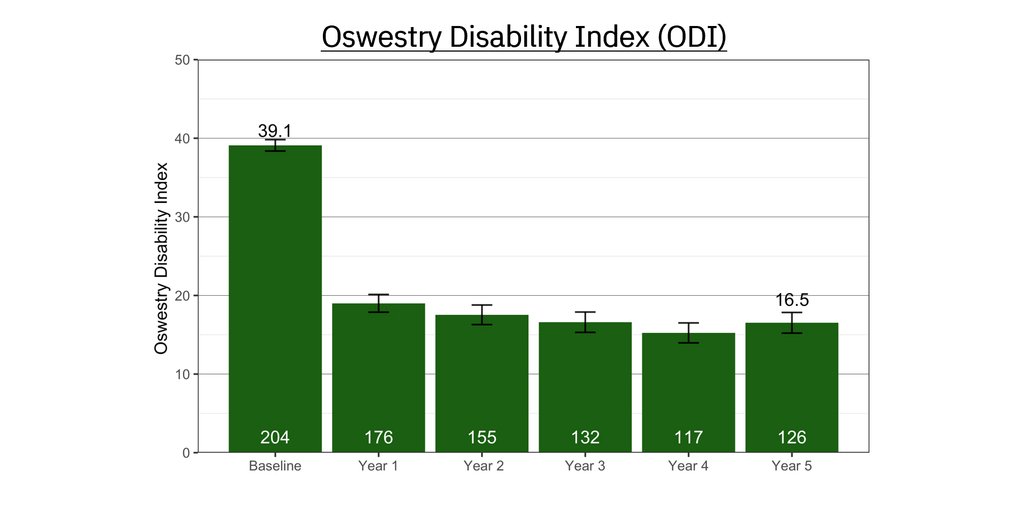
The primary endpoint of the mean improvement in Oswestry Disability Index (ODI) score between the treatment and control arms at the one-year follow-up visit (using mixed model for repeated measures (MMRM) for missing data) was statistically significant (p<0.001), with a clinically meaningful mean change in the ReActiv8 group compared to the control arm: ODI -19.7 ± 1.4 for the ReActiv8 group vs. -2.9 ± 1.4 for the control group.
All secondary endpoints showed statistically significant differences between the ReActiv8 and control arms (using MMRM for missing data), as well as clinically meaningful improvements for the ReActiv8 arm, at one year, including:
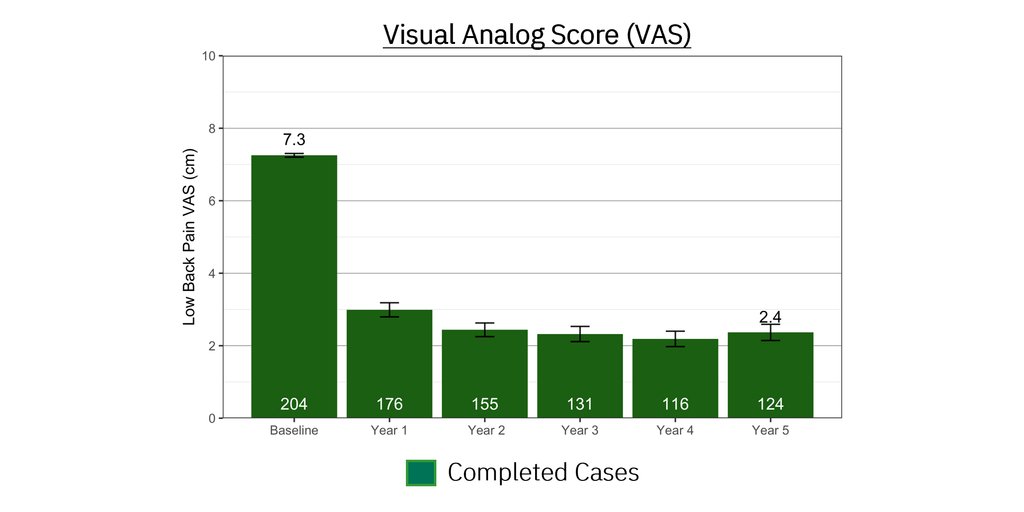
Mean improvement in back pain measured using the 11-point Numeric Rating Scale (NRS): -3.6 ± 0.2 for the ReActiv8 group vs. -0.6 ± 0.2 for the control group (p<0.001); and
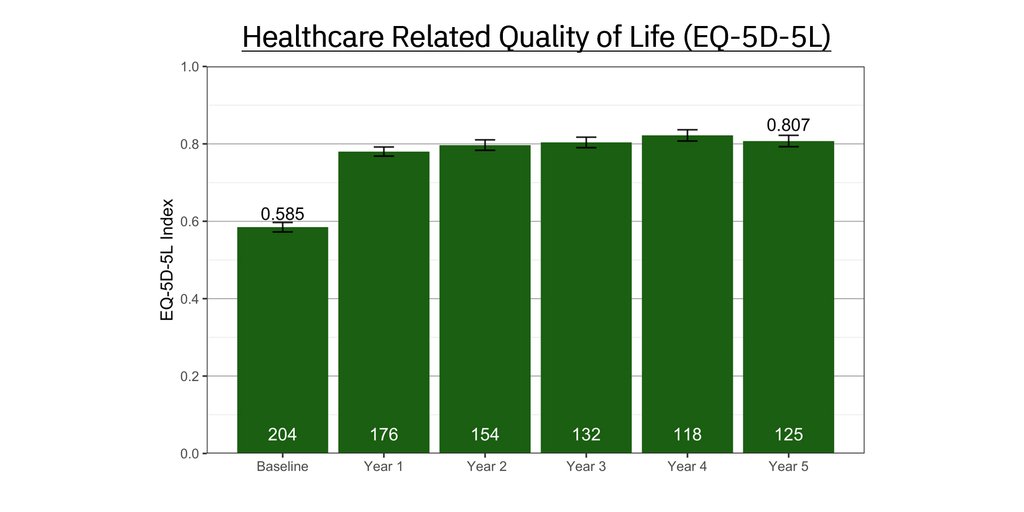
Mean improvement in healthcare related quality of life measured using the EQ-5D-5L assessment: +0.155 ± 0.012 for the ReActiv8 group vs. +0.008 ± 0.012 for the control group (p<0.001). The improvement in the ReActiv8 group resulted in a mean quality of life score approaching the average quality of life score from the overall US population.
The proportion of patients who reached the composite endpoint of ∶15-point ODI improvement and/or ≥ 50% NRS improvement and no worsening in either measure at one year was 72% in the ReActiv8 group and 11% in the control group (p< 0.001).
Pain remission, defined as NRS of ≤ 3 at one year, was observed in 52% of patients in the ReActiv8 group and in 6% of those in the control group.
The profile of related adverse events was similar to previously reported ReActiv8 studies and favourable to other neuromodulation treatment procedures.
“This patient population has historically had extremely limited options beyond temporary palliative treatments and drugs. The results in this study demonstrated that ReActiv8 Restorative Neurostimulation provided superior improvements to the lives of patients above and beyond what is currently used to treat them,” said the steering committee of the RESTORE study, Dr. Frank Schwab, Dr. Chris Gilligan, Dr. Nagy Mekhail, and Dr. Kiran Patel. “These exciting results further validate ReActiv8’s restorative mechanism of action treating multifidus dysfunction, a primary underlying cause of mechanical chronic lower back pain in certain patients. Combining these impressive results with the newly-issued ICD-10 code for multifidus dysfunction, this study further demonstrates the ability of clinicians to confidently select and treat patients with chronic mechanical low back pain who were previously very difficult to treat.”
“These high-quality data showing the treatment benefit of ReActiv8 compared to the current standard of care meaningfully add to the growing body of clinical evidence regarding ReActiv8 and firmly establishes the critical role of this therapy in treating intractable mechanical low back pain patients. We are proud to have the only commercially available device with a strong safety profile and long-term, peer-reviewed evidence supporting the rehabilitation of this severely affected patient population,” said Jason Hannon, CEO of Mainstay Medical. “We look forward to leveraging these data, along with the compelling results from our ReActiv8-B clinical trial and our numerous other studies, to further engage payers in the United States to expand commercial insurance access to this transformational therapy, which has the potential to deliver significant reductions in overall healthcare costs. I would like to thank Drs. Frank Schwab, Chris Gilligan, Nagy Mekhail and Kiran Patel for acting as our steering committee for this important study, as well as each of the enrolling sites, investigators and all of the participating patients.”
About the RESTORE Clinical Study and the Steering Committee
The RESTORE (ReActiv8 Stimulation Therapy vs Optimal Medical Management: A Randomized Evaluation) clinical study is a multi-center, prospective, randomized trial with one-way cross-over. A total of 203 patients were randomized and followed in the study at 23 leading centers in the U.S. Eligible patients were randomized to either optimized medical management or ReActiv8 Restorative Neurostimulation therapy plus optimal medical management. Patient-reported outcomes were collected at regular intervals out to the one-year primary endpoint assessment, at which time the patients in the control arm were offered implantation with the ReActiv8 system. Assessment of the patients will continue for an additional year.
The steering committee for the study consists of Dr. Frank Schwab, Chair of Orthopedic Spine Surgery at Lenox Hill Hospital and Chief of Orthopedic Spine Surgery for Northwell Health System; Dr. Chris Gilligan, Chief Medical Officer, Chief Quality Officer & Senior Vice President of Robert Wood Johnson University Hospital; Dr. Nagy Mekhail, Professor and Director of Evidence-Based Pain Management Research, Cleveland Clinic, and Professor of Anesthesiology at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University; and Dr. Kiran Patel, Director of Pain Medicine, Lenox Hill Hospital and Founder & CEO, NYC Neuromodulation Center of Excellence.
About ReActiv8®
ReActiv8 is an implantable medical device designed to treat adults with intractable chronic low back pain (CLBP) associated with multifidus muscle dysfunction, which may be evidenced by imaging or physiological testing. Candidates for ReActiv8 are patients with multifidus muscle dysfunction who have failed other forms of therapy (including pain medication and physical therapy) and are not candidates for spine surgery. ReActiv8 has received regulatory approval in several geographic areas, and is commercially available in the European Economic Area, Australia, the UK, and the US.
About Mainstay Medical
Mainstay Medical is a medical device company focused on commercializing its innovative implantable Restorative NeurostimulationTM system, ReActiv8®, for people with disabling mechanical CLBP. Mainstay Medical is headquartered in Dublin, Ireland and has subsidiaries operating in Ireland, the United States, Australia, Germany, and the Netherlands.
Further information can be found at www.mainstaymedical.com.
PR and IR Enquiries:
LifeSci Advisors, LLC
Brian Ritchie
Tel: + 1 (212) 915-2578
Email: britchie@lifesciadvisors.com
FTI Consulting (for Ireland)
Jonathan Neilan or Patrick Berkery
Tel. : +353 1 765 0886
Email: mainstay@fticonsulting.com
Mainstay Medical
Corporate Communications
Email: Media@mainstaymedical.com